FDA Commissioner Marty Makary says most medications should be available over-the-counter (OTC) unless they are unsafe, addictive, or require close medical monitoring — a sweeping vision that could reshape how Americans access and pay for medicine. Makary emphasized the agency’s intent to reform regulatory pathways this year, potentially enabling more common prescription drugs, such as nausea medications and menopause treatments, to be sold without a doctor’s prescription. This effort is driven by goals of lowering drug costs, increasing patient access, and simplifying healthcare navigation for millions of Americans.
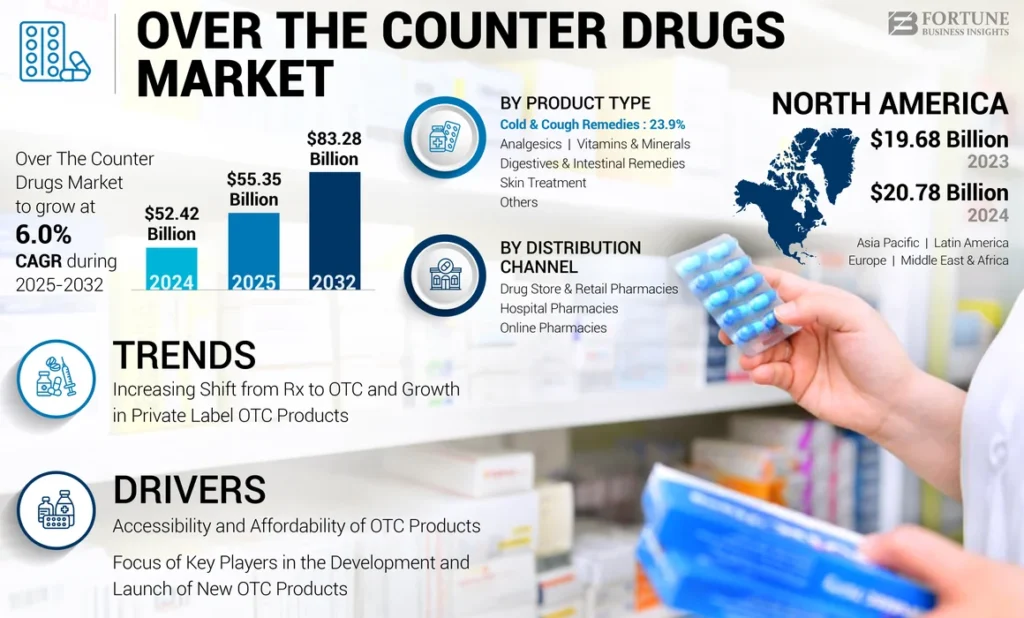
Expanding OTC Freedom: What Makary Proposes and Why It Matters Now
Makary’s bold stance — that everything should be over the counter unless clearly unsafe, addictive, or needing monitoring — underscores a broader push to dismantle what some policymakers view as unnecessary barriers to care. Traditionally, only drugs deemed safe for self-diagnosis and use without a healthcare provider’s supervision could go OTC. But under the Trump administration’s regulatory agenda, and supported by legislative changes that streamline prescription-to-OTC switch pathways, the FDA is taking steps to revisit how many drugs fall into that category.
This matters now because soaring prescription drug costs and limited access have become persistent problems in the U.S. healthcare system, particularly for chronic conditions often managed outside of acute care settings. Removing prescription requirements for safe, well-understood medicines could cut down the need for doctor visits just to renew prescriptions — a barrier for many uninsured or underinsured patients.

Why the Regulatory Shift Is Gaining Momentum
The push toward broader OTC availability isn’t occurring in a vacuum. In recent years, Congress reauthorized the Over-the-Counter Monograph User Fee Program (OMUFA II), which now requires the FDA to update guidance on transitioning prescription drugs to OTC status — including new “full switch” and “conditional switch” pathways that were previously more cumbersome.
Experts say that easing the regulatory burden for OTC switches could improve consumer autonomy, bypass insurers and pharmacy benefit managers, and boost price transparency. Makary has argued that many OTC medicines already cost less in cash than the patient’s copay for a comparable prescription drug — suggesting that wider OTC access could directly reduce out-of-pocket costs.
Supporters view this approach as a pragmatic reform, aligned with patient-centric healthcare and cost containment. For example, routine drugs for minor ailments like nausea or mild pain could become more accessible without prescriptions — much like how antihistamines and heartburn meds are already sold on retail shelves.
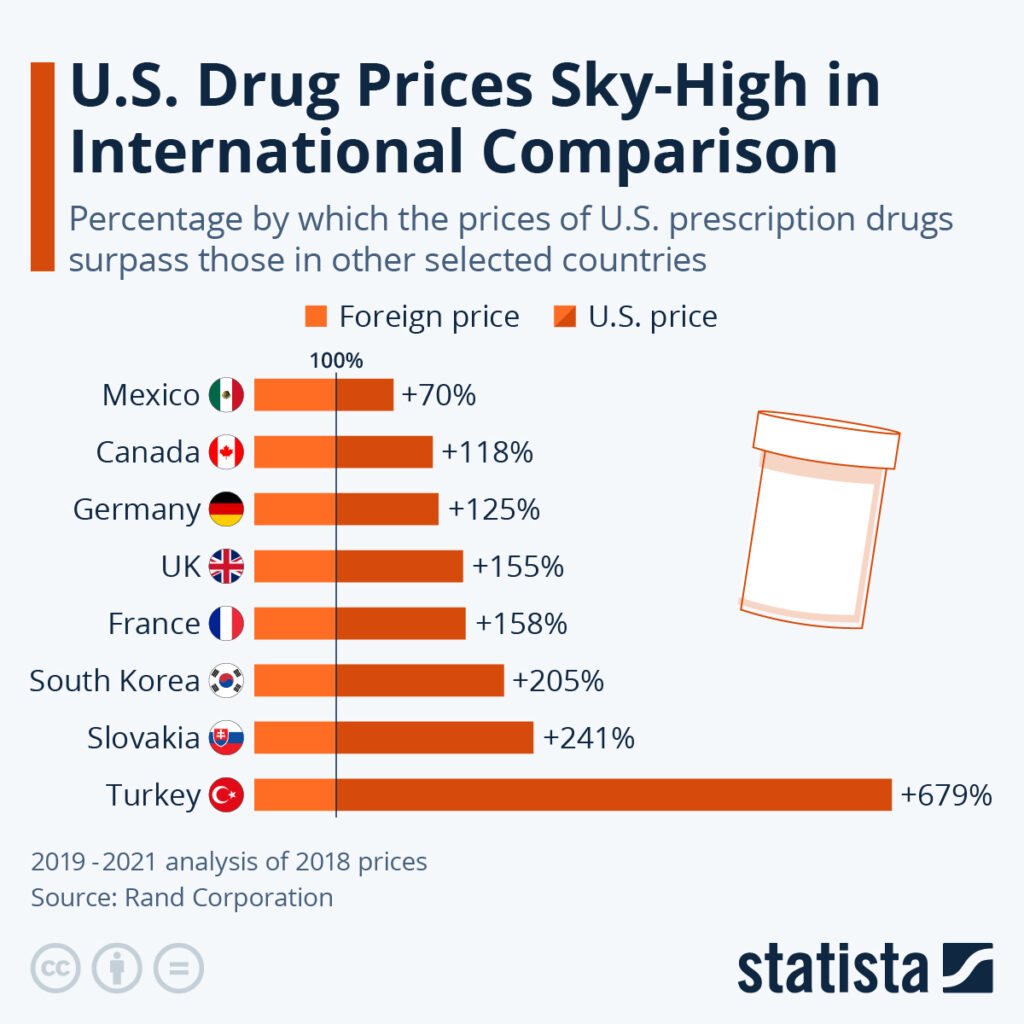
Critics Warn of Trade-Offs and Safety Concerns
Despite the potential benefits, expanding OTC access is not universally accepted. Some industry stakeholders argue that such a shift could have unintended consequences, especially if insurance coverage disappears for drugs once they lose prescription status. Unlike prescriptions, OTC medicines are typically not covered by health plans, meaning patients could pay full retail prices without reimbursement support.
Groups like the Association for Accessible Medicines have warned that removing prescription requirements might, in some cases, increase costs for patients who previously relied on insurance coverage for low-priced generic prescriptions. There’s also concern that some drugs require professional oversight — particularly those with side effects that may not be obvious to patients without lab monitoring or other clinical safeguards.

Broader Context: FDA Policy Shifts Under Makary
Makary’s push for OTC expansion is part of a larger pattern of regulatory changes at the FDA. Recent policy updates have included reducing the requirement for two rigorous studies before drug approval, which aims to speed access to new therapies while relying on advanced statistical methods and post-market surveillance.
These efforts reflect a broader ideology favoring rapid access and deregulation over more cautious, traditional review processes. While proponents see these reforms as long-overdue modernization, critics — including some former FDA scientists — argue that reducing scientific safeguards could compromise long-term safety oversight.
What This Means for Patients, Providers, and the Healthcare System
For everyday patients, a future where more drugs are available OTC could mean fewer trips to the doctor, lower overall healthcare spending, and easier access to common medicines. However, it also raises new questions about patient education, safe usage, and insurer policies — especially for individuals managing multiple conditions or complex medication regimens.
Healthcare providers and pharmacists will likely play a critical role in helping patients navigate these changes, ensuring that OTC treatments are used appropriately and that individuals still receive necessary medical oversight when required.
Makary’s proposal represents a significant shift in U.S. drug policy — one that prioritizes accessibility and affordability, but also invites debate over safety, economics, and healthcare equity. Whether this vision becomes reality will shape how Americans access medication for years to come — and why health systems, regulators, and patients alike are paying close attention.
Subscribe to trusted news sites like USnewsSphere.com for continuous updates.





