New mRNA Lung Cancer Vaccine Enters Clinical Trials Across Seven Countries
A new mRNA lung cancer vaccine has entered clinical trials across seven countries, marking a groundbreaking advancement in cancer treatment. BioNTech, the company behind this innovative vaccine called BNT116, is pioneering the use of mRNA technology—originally successful in COVID-19 vaccines—to target non-small cell lung cancer (NSCLC). This clinical trial is being conducted across 34 research sites in the United Kingdom, United States, Germany, Hungary, Poland, Spain, and Turkey, with around 130 patients participating.
Understanding the BNT116 mRNA Lung Cancer Vaccine
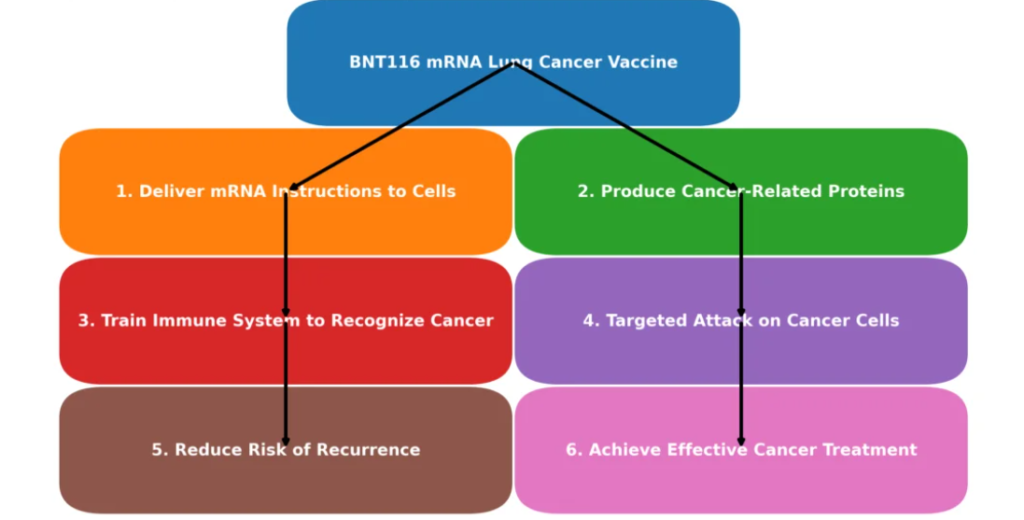
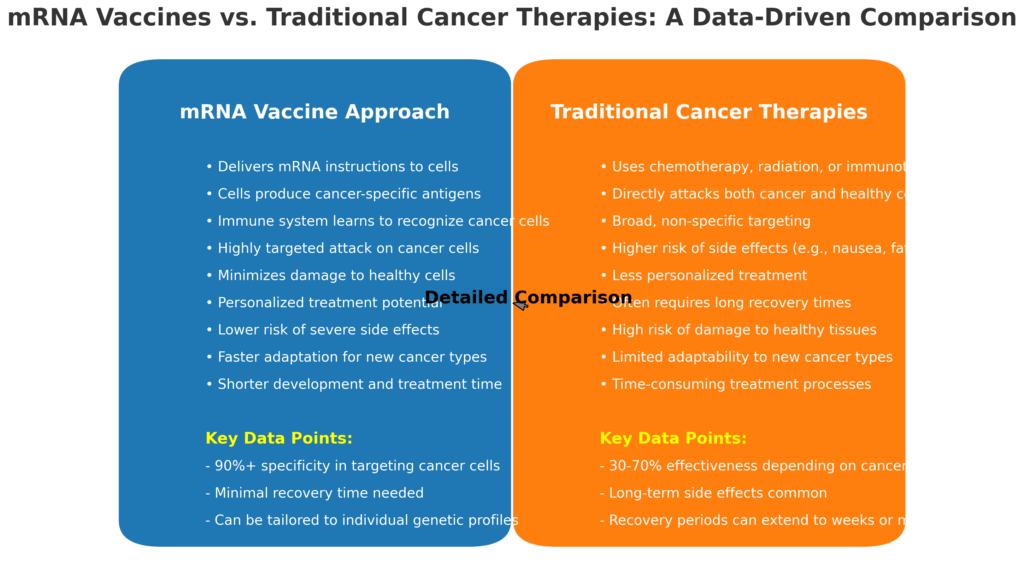
The BNT116 vaccine uses mRNA technology to train the immune system to recognize and combat cancer cells effectively. Unlike traditional chemotherapy, which often damages healthy cells, this vaccine introduces specific tumor markers associated with NSCLC, leading to a highly targeted immune response. This innovative approach aims to reduce the risk of cancer recurrence and improve long-term survival rates while minimizing side effects.
How Does the BNT116 Vaccine Work?
BNT116 operates by delivering a set of mRNA instructions to the body’s cells. These instructions help produce proteins similar to those found on cancer cells, effectively “teaching” the immune system to detect and destroy them. This mechanism not only offers a targeted treatment option but also supports a personalized approach to oncology, focusing on the unique genetic makeup of each patient’s cancer.
How the BNT116 mRNA Vaccine Works
Global Clinical Trials: Where and How They Are Conducted
The Phase 1 clinical trials for BNT116 are taking place across seven countries: the UK, USA, Germany, Hungary, Poland, Spain, and Turkey. Approximately 130 patients with various stages of NSCLC, from early to advanced or recurrent types, are involved. The trials focus on evaluating safety, tolerability, and the ability of the vaccine to elicit a robust immune response.
Table: Clinical Trial Locations and Key Focus Areas
| Country | Research Sites | Focus Areas |
|---|---|---|
| United States | 10 | Safety and Immune Response |
| United Kingdom | 5 | Advanced NSCLC Treatment |
| Germany | 6 | Early-Stage Lung Cancer |
| Hungary | 3 | Safety and Tolerability |
| Poland | 4 | Vaccine Efficacy Against Recurrence |
| Spain | 4 | Personalized Oncology Approaches |
| Turkey | 2 | Broad Immunological Impact |
Real-World Impact: What This Means for Cancer Treatment
If successful, BNT116 could transform cancer treatment by offering a less invasive and more effective therapy. The mRNA technology’s adaptability might also lead to breakthroughs in treating other cancers, highlighting its potential as a versatile tool in oncology. The medical community is particularly hopeful about how this technology can contribute to personalized medicine, tailoring treatments to individual patients based on their specific cancer profiles.
Key Data Points
- Lung cancer is the leading cause of cancer deaths worldwide.
- NSCLC accounts for approximately 85% of all lung cancer cases.
- Current 5-year survival rate for advanced NSCLC is less than 10%, emphasizing the need for new treatments like BNT116.
Conclusion: A Step Forward in Oncology
The introduction of the mRNA lung cancer vaccine BNT116 into clinical trials marks a significant milestone in cancer treatment. As the trials progress, the potential for mRNA vaccines to offer targeted, effective, and personalized cancer therapies becomes more apparent. The world watches with anticipation, hoping for a new era in oncology where lung cancer becomes not only treatable but potentially preventable.
ongoing trial updates, visit Clinical Trials Arena.