FDA Restricts COVID Vaccines to High-Risk Groups in 2025
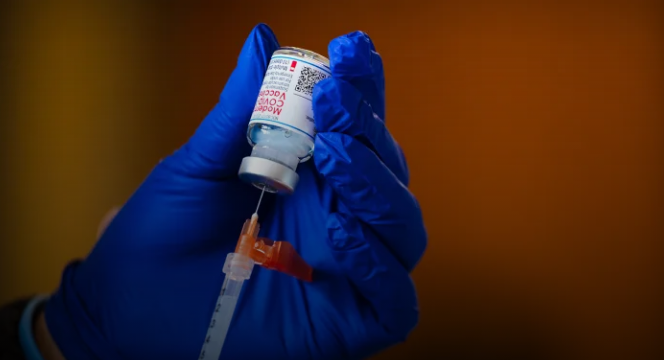
In a significant policy shift, the U.S. Food and Drug Administration (FDA) announced on May 20, 2025, that COVID-19 vaccines will now be primarily available to individuals aged 65 and older and those with high-risk medical conditions. This change aims to align the U.S. with other countries’ approaches and address concerns about vaccine efficacy in low-risk populations.

Understanding the New FDA Policy
The FDA’s updated guidelines prioritize vaccine access for seniors and individuals with conditions such as asthma, diabetes, cancer, obesity, and pregnancy. For healthy individuals under 65, the FDA now requires randomized clinical trials to demonstrate vaccine benefits before approval. This move reflects a cautious approach, emphasizing the need for robust data to support vaccine use in low-risk groups.
Dr. Vinay Prasad, director of the FDA’s Center for Biologics Evaluation and Research, stated that the agency seeks to ensure that the benefits of vaccination outweigh the risks for healthy individuals. This policy shift may lead to increased costs and limited insurance coverage for those outside the high-risk categories.
Implications for Public Health and Vaccine Access
The new policy could impact public confidence and access to vaccines. While aiming to focus resources on those most at risk, it may inadvertently create barriers for individuals who still wish to be vaccinated but do not fall into the prioritized groups. Experts express concerns that this approach might undermine efforts to maintain herd immunity and control the spread of COVID-19.
Additionally, the requirement for extensive clinical trials before approving vaccines for healthy individuals could delay the availability of updated vaccines, especially in response to emerging variants. This delay might hinder timely protection for the broader population.
Comparison with International Approaches
The FDA’s decision aligns the U.S. more closely with countries like Canada and Australia, which have adopted targeted vaccination strategies focusing on high-risk populations. These countries have emphasized the importance of protecting vulnerable groups while acknowledging the limited benefits of vaccinating low-risk individuals.
However, some public health experts argue that the U.S. context, with its unique healthcare challenges and population dynamics, requires a more inclusive approach to vaccination to ensure comprehensive protection against COVID-19.
Industry Response and Future Outlook
Vaccine manufacturers, including Pfizer and Moderna, have expressed their commitment to collaborating with the FDA to meet the new requirements. Moderna’s stock saw a significant increase following the announcement, indicating investor confidence in the company’s ability to adapt to the changing regulatory landscape.
The FDA’s advisory committee is scheduled to meet on May 22 to discuss which virus variants to target for upcoming vaccines. This meeting will be crucial in determining the direction of future vaccine development and distribution strategies.
Ethical Considerations and Public Trust
The policy shift raises ethical questions about equitable access to vaccines and the potential consequences of limiting availability to certain groups. There is concern that the new approach may exacerbate health disparities and erode public trust in the vaccination process.
Dr. Paul Offit, a member of the FDA’s advisory committee, emphasized the importance of maintaining public confidence in vaccines. He warned that restrictive policies could lead to skepticism and reduced uptake, not only for COVID-19 vaccines but also for other essential immunizations.
Conclusion
The FDA’s decision to limit COVID-19 vaccine access to high-risk groups marks a significant change in the U.S. vaccination strategy. While intended to focus resources on those most vulnerable, the policy introduces challenges related to public trust, equitable access, and timely protection against emerging variants. As the situation evolves, ongoing dialogue among policymakers, healthcare providers, and the public will be essential to navigate the complexities of pandemic response. (Time.com)
Subscribe to trusted news sites like USnewsSphere.com for continuous updates.
[USnewsSphere.com / wp.]