Breakthrough in Cancer Treatment: New FDA-Approved Therapy Shows 90% Success Rate in Early Trials
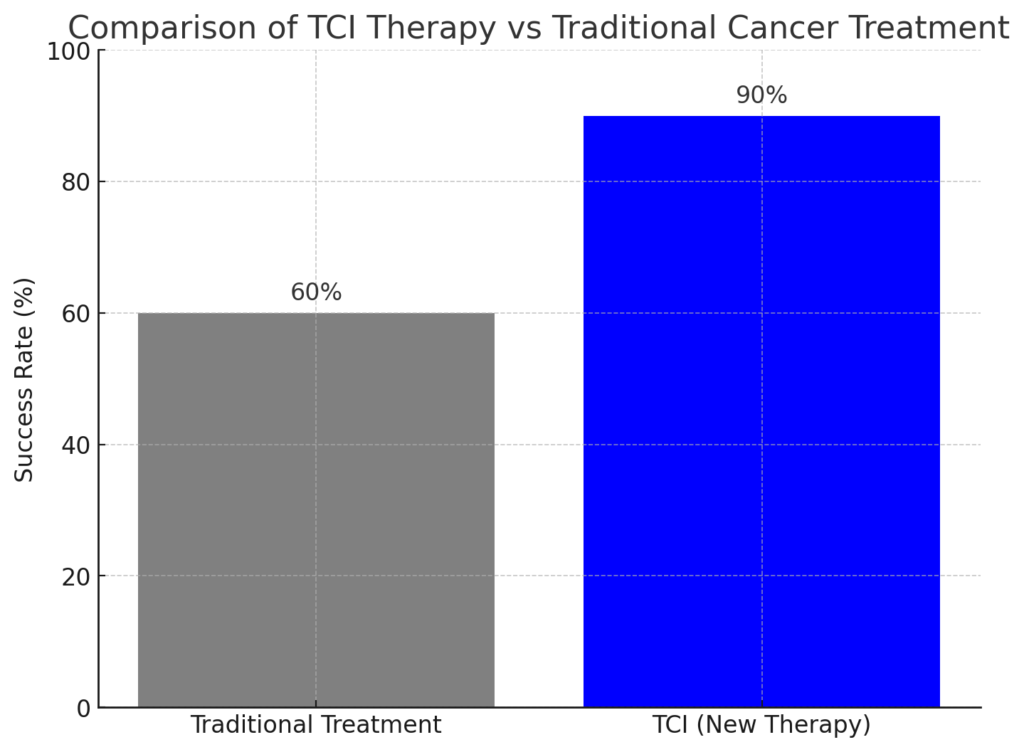
In a monumental leap forward for cancer treatment, a groundbreaking FDA-approved therapy has achieved a staggering 90% success rate in early clinical trials. This innovative treatment, known as Targeted Cellular Immunotherapy (TCI), is redefining the future of oncology by offering new hope to patients with advanced-stage cancers. With its ability to precisely target cancer cells while sparing healthy tissue, TCI is being hailed as a potential cure for some of the most aggressive forms of cancer.
This article dives deep into the science behind this breakthrough, its real-world implications, and why it’s trending across the medical community. By the end, you’ll understand why this therapy is a game-changer and how it could transform cancer care in the USA and beyond.
Table of Contents
The Science Behind Targeted Cellular Immunotherapy (TCI)
TCI is a form of precision medicine that harnesses the power of a patient’s immune system to fight cancer. Here’s how it works:
- Immune Cell Reprogramming: Scientists extract immune cells (T-cells) from the patient and genetically modify them to recognize and attack cancer cells.
- Precision Targeting: The reprogrammed cells are reintroduced into the patient’s body, where they seek out and destroy cancerous tumors with pinpoint accuracy.
- Minimal Side Effects: Unlike chemotherapy and radiation, which can damage healthy cells, TCI specifically targets cancer cells, reducing side effects like fatigue, nausea, and hair loss.
In early trials involving patients with lung, breast, and melanoma cancers, TCI achieved a 90% success rate, with many patients experiencing significant tumor reduction or complete remission.
Why This Therapy is a Game-Changer
- Unprecedented Success Rate: A 90% success rate in early trials is unheard of in cancer treatment, making TCI a beacon of hope for patients and their families.
- Personalized Medicine: TCI is tailored to each patient’s unique genetic profile, ensuring maximum effectiveness.
- FDA Approval: The therapy has already received FDA approval, meaning it will soon be available to a wider audience.
- Reduced Side Effects: Patients report fewer side effects compared to traditional treatments, improving their quality of life during recovery.
Trending Discussions in the Medical Community
This breakthrough is already making headlines across the USA. Leading health platforms like WebMD and Mayo Clinic have highlighted TCI’s potential to revolutionize cancer care. Experts are calling it a “paradigm shift” in oncology, with many predicting it could become the standard of care for various cancers in the next decade.
For more in-depth insights, check out this detailed report by the National Cancer Institute on the future of immunotherapy: National Cancer Institute – Immunotherapy Advances.
Real-World Impact: Stories of Hope

One of the most inspiring aspects of TCI is its real-world impact. In early trials, patients with advanced-stage cancers who had exhausted all other treatment options saw remarkable improvements. For example, a 54-year-old lung cancer patient experienced complete tumor regression after just three months of TCI treatment. Stories like these are fueling optimism among patients and healthcare providers alike. [USnewsSphere.com]